Abstract
Introduction: Early response assessment in AL amyloidosis relies on hematological response (HR). Typically, deep HR predicts for organ response (OR) and better overall survival (OS). However, this is not a tight relationship as each can occur independently as well. Moreover, time to OR can be variable in AL amyloidosis and is often delayed. Therefore, criteria for early assessment of treatment benefit that incorporates both HR and OR are needed.
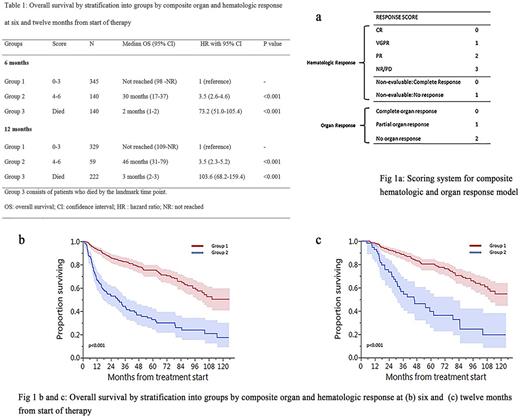
Methods: We assessed rates of OR and HR (using existing criteria) in patients with newly diagnosed AL from 2006-2015 with involvement of heart, kidney or liver at 6 and 12 month landmarks from start of therapy. A combined OR parameter was developed: complete OR, if response was achieved in all of the organs (heart, kidney, liver) involved; partial (response in at least one of the organs involved) and no response. We then combined this with HR to develop an easy to use model to risk stratify patients. (Fig 1a) Patients were given scores of 0-4 for HR at six months [0- complete response (CR), 1- very good partial response (VGPR), 2-partial response (PR), 3-no response (NR), 4-died] and 0-3 for OR (0-complete OR, 1-partial OR, 2- no OR, 3- died). Patients who had non-evaluable dFLC (difference in involved and uninvolved light chain) were given a score of 0 for CR and 1 for no response. Survival analysis was carried out using the Kaplan-Meier method. Cox regression was used to compare the predictive power of HR, OR, and a composite of both responses. Goodness-of-fit and predictive power of the individual and composite models were compared using the Akaike information criterion (AIC) and Harrell's C, respectively.
Results:
We evaluated 875 AL patients with involvement of heart (76%), kidney (66%) or liver (17%). Involvement of more than 1 of these 3 organs was seen in 48% patients. Involved LC was λ in 75%, with median dFLC of 21 mg/dL and median bone marrow plasma cells of 10%. Revised Mayo stage was 1/2/3/4, n (%): 181/183/210/223 (23/23/26/28). t(11;14) was seen in 50% (265/530) patients. Treatments received were as follows: autologous stem cell transplant based treatment, 30% (n=261); bortezomib-based chemotherapy, 26% (n=229); alkylator based therapy, 39% (n=340), immunomodulatory drugs 4% (n=34) and steroids alone or other treatments in 1% (n=11).
HR at 6 months from treatment start was evaluable in 639 patients and was: CR - 18%, VGPR - 25%, PR - 21%, NR - 14%, while 22% patients died by the six month landmark time point. OR. Rates of individual OR at 6 months were: cardiac: 17% (77/448), renal: 29% (116/407) and liver: 15% (17/111). Patients who had died by landmark time points were considered non-responders. Complete OR (response in all involved and evaluable organs) was seen in 18% (123/671), partial OR (OR in at least one organ) was seen in 9% (61/671) patients, while 51% had no OR (342/671) and 146 patients had died by the landmark.
Patients with complete OR had better OS (median: not reached) compared with partial (median: 47 months) or no OR (median: 21 months). Using the composite HR and OR model, patients were divided into 2 groups with different survival outcomes and median OS was - Group 1 (scores 0-3): not reached (NR), Group 2 (scores 4-6): 30 months. This model was validated at the twelve month landmark (HR, OR data not shown) and patients in group 1 continued to have better survival than patients in group 2 (median OS: NR vs. 46 months, p <0.001]. (Table 1, Fig 1b-c) The composite model had significantly higher predictive power (Harrel's C = 0.72, 95% CI 0.69 - 0.75) compared to the HR model (Harrel's C = 0.68, 95% CI 0.65 - 0.72, p = 0.007) and to the OR model (Harrel's C = 0.67, 95% CI 0.64 - 0.70, p <0.001). Goodness of fit of the composite model (AIC = 3201) was significantly better compared to the HR model (AIC = 3271) and the OR model (AIC = 3268) in isolation; p < 0.001 for both comparisons using the likelihood ratio test.
Conclusion: In conclusion, our study provides criteria where both hematologic and organ response can be assessed simultaneously early on in the treatment of AL amyloidosis to risk stratify patients, supporting its use as a surrogate end-point in clinical trials. This would allow for shorter duration of follow-up and enable faster completion of these studies, as well as be very useful in studies of novel therapies directed against the misfolded amyloid fibril. Given the simplicity of the model, it can also be easily integrated into daily clinical practice.
Sidana: Janssen: Honoraria. Dispenzieri: Celgene, Millenium, Pfizer, Janssen: Research Funding. Gertz: Millennium: Consultancy, Honoraria; Celgene, Novartis, Smith-Kline, Prothena, Ionis, Amgen: Honoraria. Lacy: Celgene: Research Funding. Dingli: Millenium, Takeda, Janssen, Alexion pharmaceuticals: Consultancy; Karyopharm Therapeutics: Research Funding. Kapoor: Takeda, Celgene and Amgen: Research Funding. Leung: Prothena: Consultancy. Russell: Vyriad: Equity Ownership; Imanis Life Sciences: Equity Ownership. Kumar: Celgene, Millennium/Takeda, Onyx, AbbVie, Janssen, Sanofi, Novartis, Amgen, Genentech, Merck, Oncopeptides, Roche, Skyline Diagnostics: Research Funding; Celgene, Millennium, BMS, Onyx, Janssen, Noxxon, AbbVie, Amgen, Merck, Oncopeptides, Skyline Diagnostics, Takeda: Consultancy; Skyline: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal